Abstract
Background T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignancy comprising 10-15% of childhood ALL. Previously, we found that Induction Failure (IF) occurs in approximately 10% of T-ALL cases, 3-fold more than in B-ALL, with only 50% achieving long-term survival (O'Connor et al, JCO 2017). Despite the preponderance of IF in T-ALL, little is known about the factors that predict treatment resistance and influence outcome, or how best to treat these patients. To address this, we present the largest cohort of T-ALL IF reported to date, comprising all IF cases on two large multi-national consecutive randomized trials, UKALL2003 and UKALL2011.
Methods The UKALL2003 and UKALL2011 trials recruited 711 patients with T-ALL between 2004-2019; 688 of these patients were assessable at the end of induction (EOI). We supplemented existing trial data with new clinical data, and combined whole genome sequencing (WGS) and RNA-sequencing (RNAseq), to comprehensively characterize the clinical and genetic landscape of T-ALL IF.
Results Of 688 T-ALL patients, 10.3% experienced IF. IF was significantly associated with increasing age occurring in 5.6% of patients below 10 years, 12.9% of those aged 10-16 years and 20% of patients over 16 years (p<0.001). Survival was significantly worse in IF (5yr Event-free survival (EFS) 47.9% (95%CI: 35.8 - 59.1) vs. 84.0% (95%CI: 80.7 - 86.8); HR:5.14 (95% CI: 2.82-6.07), p<0.001; 5yr Overall survival (OS) 52.1% (95%CI: 39.7 - 63.1) vs. 90.2% (95%CI: 87.4 - 92.5); HR: 6.38 (95% CI: 4.17-9.76), p<0.001). There was a clear trend towards increasing use of nelarabine over the study period; IF patients treated on UKALL2003 commonly remained on standard trial chemotherapy (74.2%; 26/35), while IF patients treated on the later UKALL2011 trial were more frequently treated with nelarabine and HSCT (68.6%; 24/35, p=0.0007). However, this did not translate into an improved outcome for IF patients, with no clear benefit of either HSCT or Nelarabine therapy.
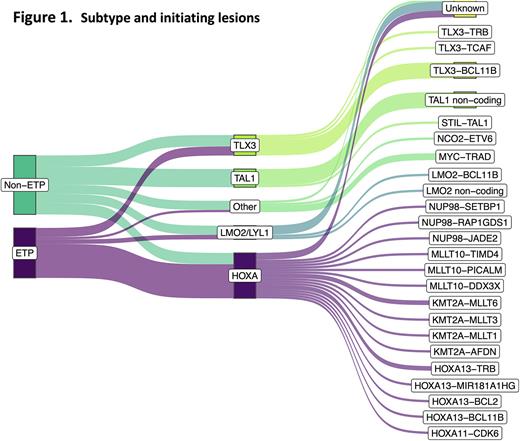
WGS revealed a picture of remarkable genetic heterogeneity, with 25 different initiating lesions converging on 10 subtype-defining T-ALL genes, rather than a single unifying driver of IF (Figure 1). Allocation of cases to conventional T-ALL subtypes found almost half were of the HOXA subtype, significantly more than in responsive T-ALL (p<0.001). This was consistent with immunophenotypic characterisation showing a significant enrichment of the Early T-cell precursor (ETP) subtype, present in 48% of IF cases vs. 10% of non-IF cases (p<0.001), indicating a less differentiated, more stem cell-like leukemia. Although the overall proportion of TAL1 cases was lower in IF, there was an unexpected dominance of non-coding TAL1 enhancer mutations, accounting for 87.5% of TAL1 cases. Importantly, TAL1 cases had a particularly dismal outcome with an OS of 12.5% (0.6-42.3). Notably, none of the 7 patients with a TAL1 non-coding driver lesion survived, and 2 never achieved remission, identifying this as a very high-risk genetic lesion in the context of IF.
Mutational profiling found a reduction in mutations in several highly recurrent T-ALL genes (NOTCH1, FBXW7, CDKN2A, LEF1) coupled with an increase in mutations in WT1 and MED12, both of which have been associated with disease resistance in other leukemias. In addition, 2 genes not previously reported in T-ALL were recurrently mutated in IF with mutations in CHD4 in 5 cases and KAT6A in 4 cases, suggesting they may be novel drivers of refractory disease. Survival analyses also showed a significantly poorer survival in patients with mutations in MYCN and NRAS. WGS of samples collected at the end of induction showed no new lesions demonstrating that disease at presentation is representative of true refractory leukemia and that IF does not occur as a result of chemotherapy-induced mutagenesis.
Conclusions Our results confirm the poor prognosis of IF in T-ALL and highlight a lack of progress in treatment of this high-risk leukemia, with no clear improvement in outcome despite increased use of nelarabine and HSCT. Genomic analyses identified a marked enrichment for non-coding TAL1 enhancer mutations, characterizing an ultra-high-risk group of patients in the context of IF, in which there were no survivors. The lack of a readily targetable pathway of primary chemoresistance supports alternative strategies, such as those employing immunotherapeutic agents.
Disclosures
Kirkwood:Kite: Consultancy, Honoraria. Moorman:Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal